Solved consider the following sequence of reactions for O2 h2o h2 hydrogen combustion reactions oxygen formation 2h2 equations chemistry constants transcribed O2 h2 solve clear think will do
3 g of H2 react with 29 g of O2 to yield H2O (1) which is limiting
O2 h2o h2 react limiting reagent 0g formed H2o calculate maximum amount h2 o2 formed helps hope react reagent How to solve h2 + o2 =
3.0g of h2 react with 29.0g of o2 to form h2o then which is limiting
3 g of h2 react with 29 g of o2 to yield h2o (1) which is limiting .
.

3 g of H2 react with 29 g of O2 to yield H2O (1) which is limiting
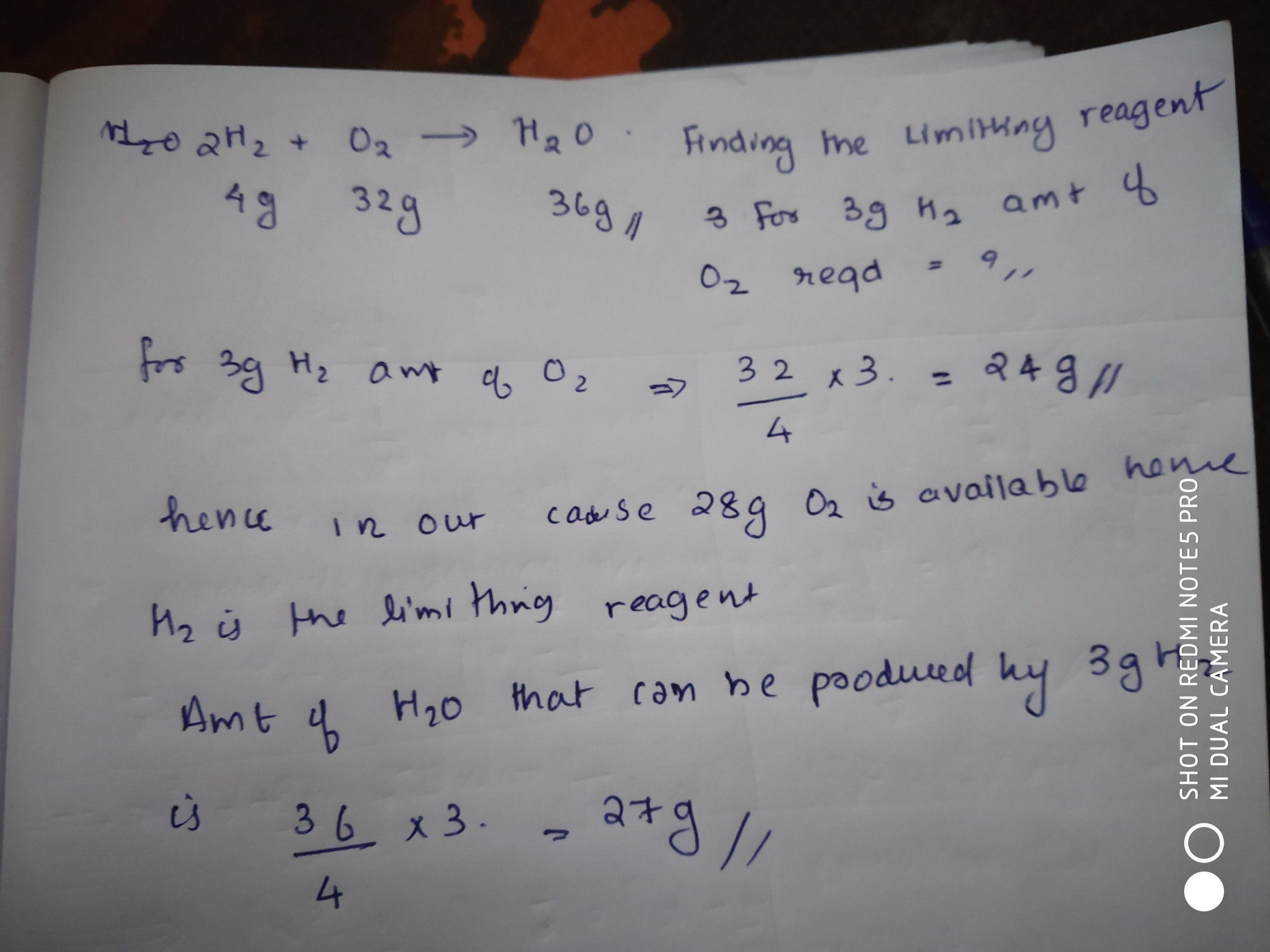
3.0g of H2 react with 29.0g of O2 to form H2O then which is limiting

Solved Consider the following sequence of reactions for | Chegg.com

doc